Hello, and welcome back to Beyond NERVA! I would like to start by apologizing for the infrequency in posting recently. My wife is currently finishing her thesis in wildlife biology (multispecies occupancy, or the combination of statistical normalization of detection likelihood with the interactions between various species in an ecosystem to determine inter-species interactions and sensitivities), which has definitely made our household more hectic in the last few months. She should defend her thesis soon, and things will return to somewhat normal afterward, including a return to more frequent posting.
Today, we return to radioisotope thermoelectric generators (RTGs), which have once again been in the news. This time, the new is on a happier note than the passing of one of the pioneers in the field: the refining of a different type of fuel for RTGs: Americium 241. This work was done at the United Kingdom’s National Nuclear Laboratory Cumbria Lab by a team including personnel from the University of Leicester, and announced on May 3rd. This material was isolated out of the UK’s nuclear weapons stockpile of plutonium for nuclear warheads, which is something that we’ll look at more in the post itself.
A quick note on nomenclature and notation, since this is something that varies a bit: the way I learned to notate isotopes of an element (or nuclei of a given element with different numbers or neutrons) is as follows, and is what I generally use. If the element is spelled out, the result is [Element name][atomic mass of the nucleus](isomer state – if applicable); if it’s abbreviated it’s [atomic mass](isomer)[element symbol]. An isomer shows the energetic state of the nucleus: if gamma rays are absorbed by the nucleus, then a nucleon can jump to a higher energy state, just as electrons do for lower-wavelength (and lower energy) photons, and for this post it may not matter, but will come up with this element in the future. This means that Plutonium 238 will become 238Pu, and Americium 242(m) becomes 242(m)Am – I chose that because it’s a long-lived isomer that we will return to at some point due to its incredible usefulness in astronuclear design and reactor geometry advantages.
In this post, we’ll take a brief look at the fuels we currently use, as well as this “new” fuel, and one or two more that have been used in the past.
Radioisotope Power Source Fuels: What Are They, and How Do they Work?
Radioisotopes release energy in proportion to the instability of the isotope of whatever element is being used. This level of instability is a very complex issue, and how they decay largely is determined by where they fall in relation to the Valley of Stability, or the region in the Chart of the Isotopes where the number of protons and the number of neutrons balances the strong nuclear and electroweak forces in the nucleus. We aren’t going to go into this in too much detail, but the CEA did an excellent video on this topic which is highly recommended viewing if you want more information on the mechanics of radioactive decay and its different manifestations:
[youtube https://www.youtube.com/watch?v=UTOp_2ZVZmM&w=560&h=315]
For our purposes, the important things to consider about radioactive decay are: how much energy is released in a given amount of time, and how easy is that energy to harness into kinetic energy, and therefore heat, while minimizing the amount of unharnessable radiation that could potentially damage sensitive components in the payload of the spacecraft. The amount of energy released in a given time is inversely proportional to the half-life of the isotope: the shorter the half-life, the more energy is released in a given time, but as a consequence the energy is being released at a faster rate and therefore the fuel will produce useful energy levels for a shorter period of time. How much of that energy is useful is determined by the decay chain of the isotope in question, which shows the potential decay mechanisms that the isotope will go through, how much energy is released in each decay, and how what the subsequent “daughter” isotopes are – with their decay mechanisms as well. The overall energy release from a particular isotope has to take all of these daughters, granddaughters, etc into consideration in order to calculate the total energy release, and how useful that energy is. If it’s mostly (or purely) alpha radiation, that’s ideal since it’s easily converted to kinetic energy, and each decay releases a proportionally larger amount of energy on average due to the high relative mass of the alpha particle. Beta radiation is not as good, but still easily shieldable (which converts the energy into kinetic energy and therefore heat), and so while not as ideal for heating is still acceptable.
For an RTG designer, the biggest problem (in this area at least) is gamma radiation. Not only is this very hard to convert into useful energy, but it can damage the payload and components of a spacecraft. This is because of the way gamma radiation interacts with matter: the wavelength is so short that it is absorbed most efficiently by very dense elements with a high number of nucleons (protons and neutrons), after which that nucleon jumps to a higher energy state (an isomer), and usually just spits it back out at a lower energy state. This process is repeated until there isn’t enough energy in the short wavelength photon to actually do anything meaningful.
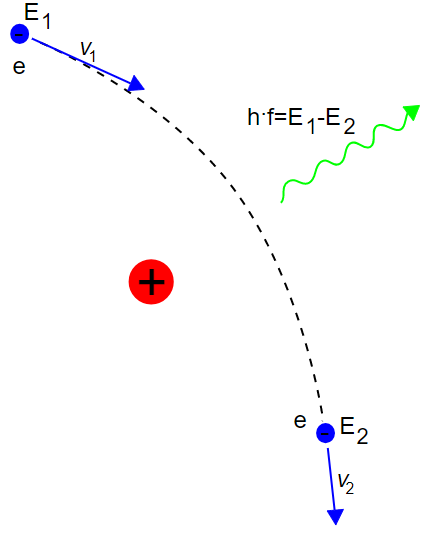
Unfortunately, radioactive decay isn’t the only way to produce gamma rays, you also have to deal with one of the key concepts in radiation shielding which always ties my fingers in knots whenever I try and type it (my tongue just says “nah, not even going to try”): Brehmsstrahlung. This is also known by the name “braking radiation,” and is close to the concept of “cyclotron radiation.” Basically, when a massive, high energy particle is diverted from its original course, two things happen: it slows down, and that slowing down means that energy has to be conserved somehow. In the case of quantum mechanics, the elastic collisions between the particle and whatever force acts on it (in practice the electromagnetic field it interacts with) creates kinetic energy (heat) if it hits a particle, and in every case (including magnetic containment of the particle) a photon is produced. This photon’s wavelength is proportional to the energy the particle originally has, the degree that its direction is changed, and how much energy is lost through mechanisms other than elastic collisions with other nucleons – and it’s never able to be fully slowed by elastic collisions for very complex quantum mechanical reasons. The amount converted to brehmstrahlung is proportional to the initial velocity of the particle in the case we’re talking about (magnetic containment and diversion of radiation isn’t the subject of this blog post after all). This means that if you have a high energy alpha particle emitter, and a low energy alpha emitter, the high energy alpha emitter will create more gamma radiation, meaning that it’s more problematic in terms of energy efficiency of a radioisotope for heat production.
Another concern is what chemical form the radioisotope is used in the fuel element itself. This defines a number of things, including how well any heat produced is transmitted to where it’s useful (or if the heat isn’t transferred efficiently enough, the thermal failure point of the fuel), what kind of nuclear interactions will occur within the fuel, the chemical impact of the elemental composition of the fuel changing through the radioactive decay that is the entire point of using these materials, how much the particles are slowed within the fuel itself (and any subsequent brehmsstrahlung), how much of the radiation that comes from the radioisotope decay is shielded by the fuel itself, and a whole host of other characteristics that are critical to the real-world design of a radioisotope power source.
Now that we’ve (briefly) looked at how radioisotopes are used as power sources, let’s look at what options are available for fueling these systems, which ones have been used in the past, and which ones may be used in the future.
How Are Radioisotopes Used in Space?
The best known use of radioisotopes in space is the use of radioisotope thermoelectric generators, RTGs. RTGs are devices that use the thermoelectric effect, paired with a material containing an isotope going through radioactive decay, to produce electricity. While these are the most well known of the radioisotope power sources, they aren’t the only ones, and in fact they are made up of an even more fundamental type of power source: a radioisotope heating unit, or RHU.
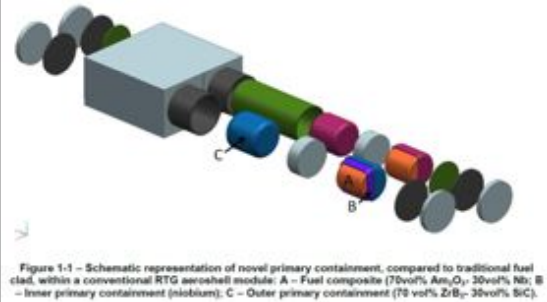
ESA RHU Exploded diagram, Summerer 2013. Apologies for poor image quality.
These RHUs are actually more common than RTGs, since often the challenge isn’t getting power to your systems but keeping them from freezing in the extreme cold of space (when there isn’t direct sunlight, in which case it’s extreme heat which is the problem). In that case, a small pellet of radioisotope is connected to a heat transfer mechanism, which could be simple thermal conduction through metal components or the use of heat pipes, which use a wick to transfer a working fluid from a heat source to a cold sink through the boiling and then condensation of the working fluid. Heat pipes have become well known in the astronuclear community thanks to the Kilopower reactor, but are common components in electronics and other systems, and have been used in many flight systems for decades.
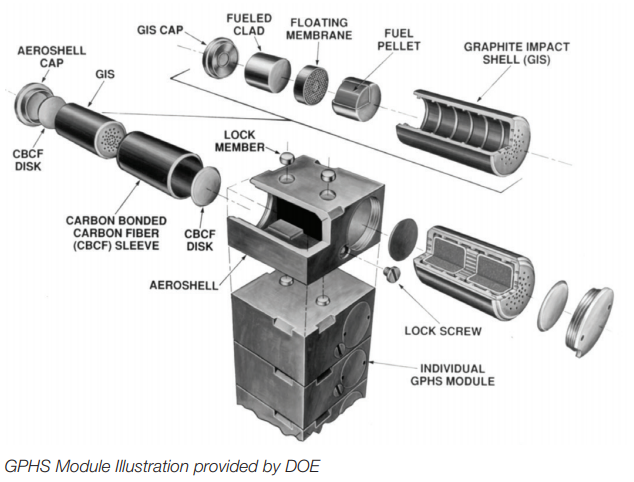
NASA GPHS design
RTGs use RHUs as well, as the source of their heat to produce electricity. In fact, the design of a common RHU for both RTG and spacecraft thermal management requirements was a focus of NASA for years, resulting in the General Purpose Heating Unit (GPHU) and its successor variations by the same name. This is important to efficiently and reliably manufacture the radioisotope fuel needed for the wide variety of systems that NASA has used in its spacecraft over the decades. While RTGs are the focus of this technology, we aren’t interested in either the power conversion system or the heat rejection system that make up two of the four main systems in an RTG, so we won’t delve into the details of these systems in particular. Rather, our focus is on the RHU radioisotope fuel itself, and the shielding requirements that this fuel mandates for both spacecraft and payload functionality (the other two major systems of an RTG). Because of this, for the rest of the post we will be discussing RHUs rather than RTGs for the most part (although mentions of RTG implications will be liberally scattered throughout).
Another potential use for radioisotopes in space seems to be something that is rare in discussions of space systems, but is common on Earth: as a source of well-characterized and -understood radiation for both analysis and manufacture of materials and systems. Radioisotope tracking is common in everything from medicine to agriculture, and radioactive analysis is used on everything from ancient artifacts to modern currency. The ISS has had experiments that use mildly radioactive isotopes to analyze the growth of living beings and microbes in microgravity environments, for instance, a very common use in agriculture to analyze nutrient uptake in crops, and a variation of a technique used in medicine to analyze everything from circulatory flow to tumor growth in nuclear medicine. X-ray analysis of materials is also a common method used in high-end manufacturing, and as groups such as Made in Space, Relativity Space and Tethers Unlimited continue exploring 3d printing and ISRU in microgravity and low gravity environments, this will be an invaluable tool. However, this is a VERY different subject, and so this will be where we leave this biological analysis and technological development technology and move to the most common use of radioisotopes: to provide heat to do some sort of work.
RHU Fuel: The Choices Available, and the Choices Made
Most RHUs, for space applications at least, are made from 238Pu, which is an isotope of plutonium that is not only not able to undergo fission, but in fairly minute quantities will render Pu meant for nuclear weapons completely unusable. In the early days of the American (and possibly Soviet) nuclear weapons program, small amounts of this isotope were isolated from material meant for nuclear weapons, but as time went on and the irradiation process became more efficient for producing weapons (which is very different from producing power), the percentage of 238Pu dropped from the single digits to insignificant. By this time, though, the Mound Laboratories in Miamisburg, Ohio had become very interested in the material as a source of radioactive decay heat for a variety of uses. These uses ranged from spacecraft to pacemakers (yes, pacemakers… they were absolutely safe unless the person was cremated, and the fact that the removal of said pacemakers couldn’t be guaranteed is what killed the program). This doesn’t mean that they didn’t investigate the rest of what we’ll be looking at as well, but much of their later focus was on 238Pu.
The advantages of 238Pu are significant: it only undergoes alpha decay with an exceptionally small chance of spontaneous fission (which occurs in the vast majority, if not all, isotopes of uranium and above on the periodic table), it has a good middle-of the road half-life (87.84 years) for long term use as a power source for missions lasting decades (as the majority of outer solar system missions require just to get tot he target destination and provide useful science returns), it has a good amount of energy released during alpha decay (5.59 MeV), and its daughter – 234U – is incredibly stable, with a half-life so long that it basically won’t undergo any significant decay until long after the fuel is useless and the spacecraft has fulfilled its mission centuries ago (245,500 years). The overall radiation power for 238Pu is 0.57 W/g, which is one of the best power densities available for long lived radioisotopes.
Additionally, the fuel used PuO2, is incredibly chemically stable, and when 238Pu becomes 234U, those two oxygen atoms are easily able to reattach to the newly formed U to form UO2 – the same fissile fuel form used in most nuclear reactors (although since it’s 234 rather than 235, it would be useless in a reactor), which is also incredibly chemically stable. Finally, the fuel itself is largely self-shielding once encased in the usual iridium clad to protect the fuel during handling and transport, meaning that the minimal amount of gamma radiation coming off the power source is only a major consideration for instruments looking in the x-ray and gamma bands of the EM spectrum causing noise, rather than significant material or electronic degradation.
238Pu is generated by first making a target of 237Np, usually through irradiation of a precursor material in a nuclear reactor. This target is then exposed to the neutrons produced by a nuclear reactor for a set length of time, and then chemically separated into 238Pu. Due to the irradiation time and energy level, the final result is almost pure 238Pu (close enough for the DOE and NASA’s purposes, anyway), which can then be turned into the ceramic pellet and encased in the clad material. This is then mated to the system that the spacecraft will use the power source for, usually just before spacecraft integration into the launch vehicle. Due to the rigid and strict interpretations of radiation protection, this is an incredibly complex and challenging process – but one that is done on a fairly regular basis. The supply of 238Pu has been a challenge from a bureaucratic perspective, but a recent shakeup of the American astronuclear industry due to a GAO report released last year offers hope for sufficient supply for American flight systems.
This is far from the only radioisotope source used for RHUs – in fact, it wasn’t even the first. Many early US designs used strontium 90, as did many Soviet designs. The first RTG to fly, SNAP-3, used this power source, as did multiple nautical navigation buoys, Soviet lighthouses along their northern coast, and many other systems. This isotope has a half-life of 28.79 years, which means that it’s more useful for shorter-lived systems than 238Pu, but is still a long enough half-life that it’s still a useful radioisotope source. The disadvantage is that it decays via beta decay (at 546 KeV), to 90Y, which is less efficient in converting the radioactive decay into heat. However, this isotope goes through another decay as well. 90Y only has a 2.66 day (!) half-life, ejecting a 2.28 MeV beta particle, resulting in a final decay product of 90Zr, which is radiologically stable. This means that the total energy release is 2.826 MeV, through two beta emissions. The overall energy release in the initial decay is attractive, at 0.9 W/g, however, so either sufficient shielding (of sufficiently high thermal conductivity) to convert this beta radiation into kinetic energy, or a different power conversion system than one that is thermally based is a potential way to increase the efficiency of these systems.
Finally, we come to the one that scares many, and has a horrible reputation in international politics, polonium 210. This is most famous for being used as a poison in the case of Alexander Litvinenko, a Russian defector who was poisoned with Po in his tea (and a whole lot of other people being exposed), but much of the effectiveness is due to chemical toxicity, not radiotoxicity. 210Po has an incredibly short half-life, of only 138.38 days. This is only acceptable for short mission times, but the massive amount of heat generated is still incredibly attractive for designers looking for very high temperature applications. Designs such as radioisotope thermal rockets (where the decay heats hydrogen or other propellant, much like an NTR driven by decay rather than a reactor) that have their efficiency defined purely by temperature can gain significant advantages thanks to this high decay energy. 210Po decays via alpha decay into lead 206, a stable isotope, so there are no complexities from daughter products emitting additional radiation, and a 5.4 MeV alpha particle carries quite a lot of energy.
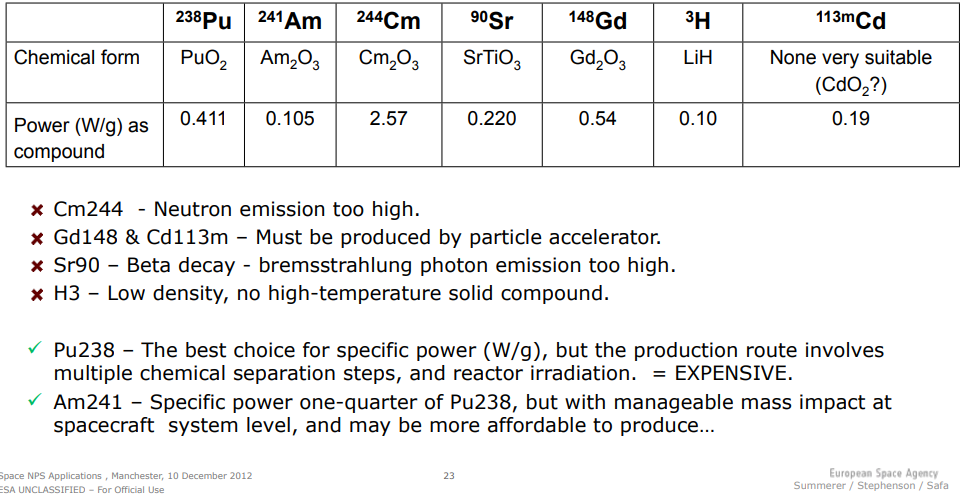
Other isotopes are also available, and there’s a fascinating table in one of my sources that shows the ESA decision process when it comes to radioisotope selection from 2012:
241Am: The Fuel in the News
Americium-241 is the big news, however, and the main focus of this post. 241Am is produced during irradiation of uranium, which goes through neutron capture during irradiation to 241Pu, which is one of the isotopes that degrades the usefulness of weapons-grade 239Pu. It decays in a very short time (14 day half-life) into 241Am, which is not useful as weapons-grade material, but is still useful in nuclear reactors. According to Ed Pheil, the CEO of Elysium Industries, the Monju sodium cooled fast reactor in Japan faced a restart problem because the 241Pu in the reactor’s fuel decayed before restarting the reactor, causing a reactivity deficit and delaying startup.
241Am decays through a 5.64 MeV alpha emission into 237Np, which in turn goes through a 4.95 MeV alpha decay with a half-life of 2.14 x 10^6 years. This means that the daughter is effectively radiologically stable. With its longer half-life of 433 years (compared to 88 years for 238Pu), 241Am won’t put out as much energy at any given time compared to 238Pu fuel, but the difference in power output as the mission continues will allow for a more steady power supply for the spacecraft. A comparison of beginning of life power to 20 year end of life power for a 238Pu RTG shows a reduction in power output of about 15%, compared to only about 3.5% for 241Am. This allows for more consistent power availability, and for extremely long range or long duration missions a greater amount of power available. However, in the ESA design documentation, this extreme longevity is not something that is examined, a curious omission on first inspection. This could be explained by two factors, however: mission component lifetime, which could be influenced by multiple factors independent of power supply, and the continuing high cost of maintaining a mission control team and science complement to support the probe.
Depending on what power level is needed (more on that in the next section about the ESA RTG design), and how long the mission is, the longer half-life could make 241Am superior in terms of useful energy released compared to 238Pu, and is one of the reasons ESA started looking at 241Am as the main focus of their RTG efforts.
Why the focus on weapons material in the description of production methods? Because that’s where the UK’s National Nuclear Laboratory gained their 241Am in the latest announcement. The NNL is responsible for production of all 241Am for Europe’s RTG programs, but doesn’t HAVE to produce the material from weapons stockpiles.
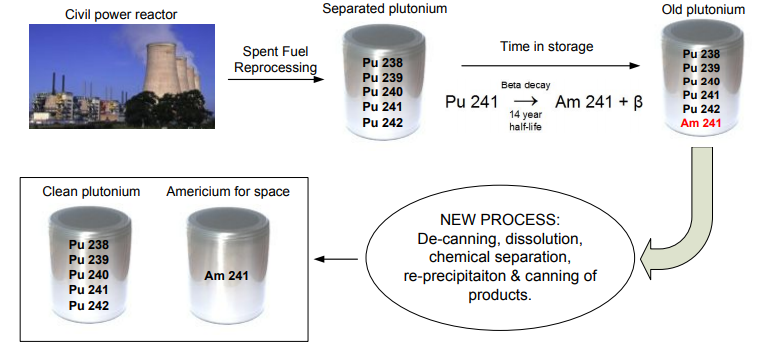
The EU reprocesses their fuel, unlike the US, and use the Pu to create mixed-oxide (MOX) fuel. If the Pu is chemically separated from the irradiated fuel pellets, then allowed to decay, the much shorter half-life of 241Pu compared to all of the others will lead to the ability to chemically separate the 241Am from the Pu fuel for the MOX. This could, in theory, allow for a steady supply of 241Am for European space missions. As to how much 241Am that would be available through reprocessing, this is a complex question, and one that I have not been able to explore sufficiently to give a good answer to how much 241Am would be available through reprocessing. Jaro Franta was kind enough to provide a pressurized water reactor spent fuel composition table, which provides a vague baseline:
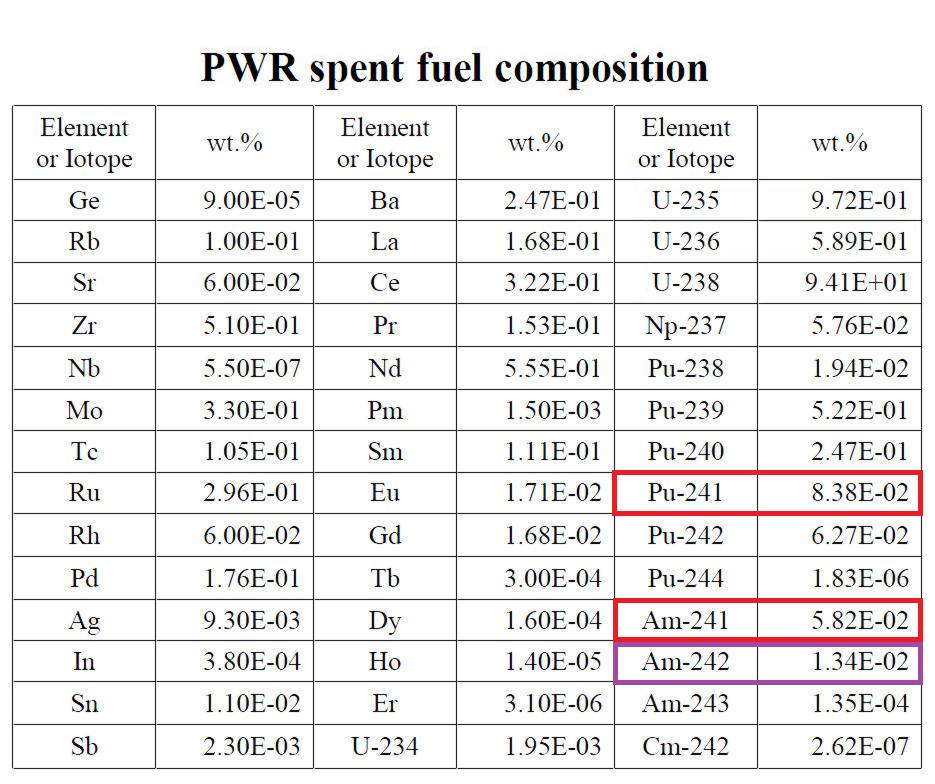
However, MOX fuel generally undergoes higher burnup, and according to several experts the Pu is quickly integrated into fuel as part of the reprocessing of spent fuel. This could be to ensure weapons material is not lying around in Le Hague, but also prevents enough of a decay time to separate the 241Am – plus, as we see in 238Pu production, where the materials are fabricated in one place, separated in another, and made into fuel in a third, Le Hague and the Cumbria Laboratory are not only in different locations but different countries, and after this process the Pu is even more useful for weapons, this bureaucratic requirement makes the process of using spent nuclear fuel for 241Am production an iffy proposition at best. However, according to Summerer and Stephenson (referenced in one of the papers, but theirs is behind a paywall) the economical separation of 241Am from spent civilian fuel can be economical (I’m assuming due to the short half-life of 241Pu), so it seems like the problem is systemic, not technical.
241Am is used as RHU fuel in the form of Am2O3. This allows for very good chemical stability, as well as reasonable thermal transfer properties (for an oxide). This fuel is encased in a “multilayer containment structure similar to that of the general-purpose heat source (GPHS) system,” with thermal and structural trade-offs made to account for the different thermal profile and power level of the ESA RTG (which, as far as I can tell, doesn’t have a catchy name like “GPHS RTG” or “MMRTG” yet). Neptunium oxide most often takes the form of NpO2, meaning that a deficit of oxygen will occur over time in the fuel pellet. The implications of this are something that I am completely unable to answer, and something that is definitely distinct from the use of 238Pu, which then becomes 234U, both of which have two oxygen atoms in their most common oxide state. However, considering there’s a stoichiometric mismatch between the initial material, the partially-decayed material, and the final, fully-degenerate state of the fuel element. I know just enough to know that this is far from ideal, and will change a whole host of properties, from thermal conductivity to chemical reactivity with the clad, so there will be (potentially insignificant) other factors that have impacts on fuel element life from the chemical point of view rather than the nuclear one.
ESA’s RTG: the 241Am RTG
ESA has been interested in RTGs for basically its entire existence, but for reasons that I haven’t been able to determine for certainty, they have not investigated 238Pu production for their RTG fuel to any significant degree for that entire time. Rather, they have focused on 241Am. This comes with a trade-off: due to the longer half-life, at any given time less energy is available per unit mass (0.57 W/g for 238Pu vs 0.1 W/g for 241Am), but as previously noted there are a couple of threshold points at which this longer half-life is an advantage.
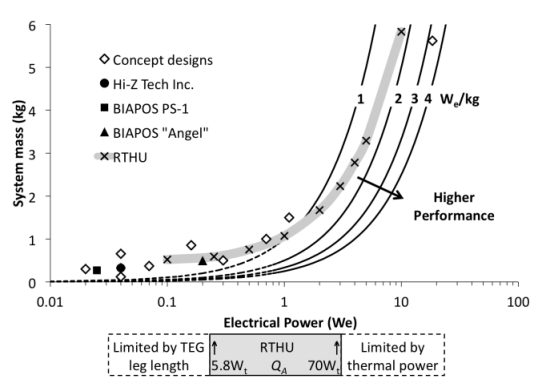
The first advantage is in mission lifetime (assuming the radiochemistry situation is of minimal concern): the centuries-long half-life could allow for unprecedented mission durations, where overall mass budget for the power source is of less concern in mission design than longevity of the mission. The second comes to when a very small amount of power is used, and this is the focus of ESA’s RTG program. The GPHS used in American systems produces 250 Wt at the time of manufacture in no more than 1.44 kg. Sadly, it’s very difficult to determine what the ESA fuel element’s mass and specific composition, so a direct comparison is currently impossible. Based on a 2012 presentation, there were two parallel options being explored, with different specific power characteristics, but also different thermal conductivity – and more efficient thermal transport. One was the use of CERMET fuel, where the oxide fuel is encapsulated in a refractory metal (which was unspecified), manufactured by spark plasma sintering. This is a technology that we’ve examined in terms of fissile fuel rather than radioisotope fuel, but many of the advantages apply in both cases. In addition, the refractory metal provides internal brehmstrahlung shielding, potentially offsetting the need for robust clad materials, but this is potentially offset by the need to contain the radioisotope in the case of a launch failure, which requires a mechanical strength that may ensure that the reduction in radiation shielding requirements are rendered effectively moot. The second was a parallel of American RHU design, using a multi-layered clad structure, using refractory metals, carbon-based insulators, and carbon-carbon insulators. This seems to be the architecture which was ultimately chosen, after a contract with Areva TA in France and other partners (which I have not been able to find documentation on, if you have that info please post it in the comments below!).
ESA has two RTG designs that they’ve been discussing over the last two decades: an incredibly small one, of only 1 W of electricity, and a larger one, in the 10-50 We range. These systems are similar in that they are both starting from the same design philosophy, but at the same time different materials and design tradeoffs were required for the two systems, so they have evolved in different directions.

The 10-50 We RTG combines the 241Am RHU with a composite clad, surrounded by a bismuth telluride thermocouple structure. This is very similar to lead telluride TE converters in many ways, but is more efficient in lower operating temperatures. PbTe is also under investigation, developed and manufactured by Fraunhofer IPM in Germany, but BiTe seems to be the current technological forerunner in ESA RTG development. This seems to be commercially available, but sadly based on the large number of thermopiles (another name for TE converters) available, it is not clear which is being used.
The 1-1.5 We RTG is one that doesn’t seem to be explored in depth in the currently published literature. This power output is useful for certain applications, such as powering an individual sensor, but is a much more niche application. Details on this design are very thin on the ground, though, so we will leave this design at that and move on to the experiment performed – again, very little is available on the specifics, but the experiment WAS described in previous papers.
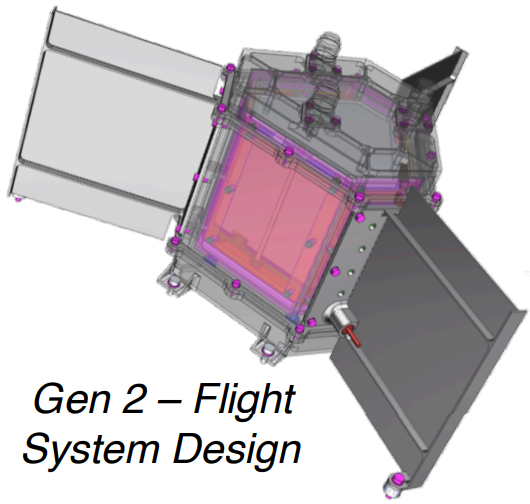
This design went through an evolution into the model seen in the public announcement video. This is called the Gen 2 Flight System Design, and likely was introduced sometime in the 2015 timeframe. At the same time, the Radioisotope Heating Unit itself went to TRL 3.
On the fuel element fabrication side, after 241Am was selected in 2010 two phases of isotope production occurred. The first was from 2011 to 2013, and the second from 2013 to 2016.
This was the first test of that batch of RTG fuel to produce electricity, which is a major achievement, for which the entire team should be congratulated.
The Experiment in the News: What is All the Fuss Actually About?
Sadly, there’s no publicly available information about the Am-fueled test in the news. The promotional video and press release from NNL/UL provided only two pieces of information: 241Am fuel was used, and they lit a light bulb. No details about how much 241Am, its fuel form or clad, the thermocouples used, the power requirements of the light bulb… this was meant for maximum viral distribution, not for conveying technical information.
The best way to look at the test is by looking at preceeding tests. RTG design from the ground up takes time, and in the case of ESA and the NNL, this process was only funded for the last ten years. They have had to pick a fuel type, continue to go through selection on thermocouple type, and will be working to finalize the design of their flight system.
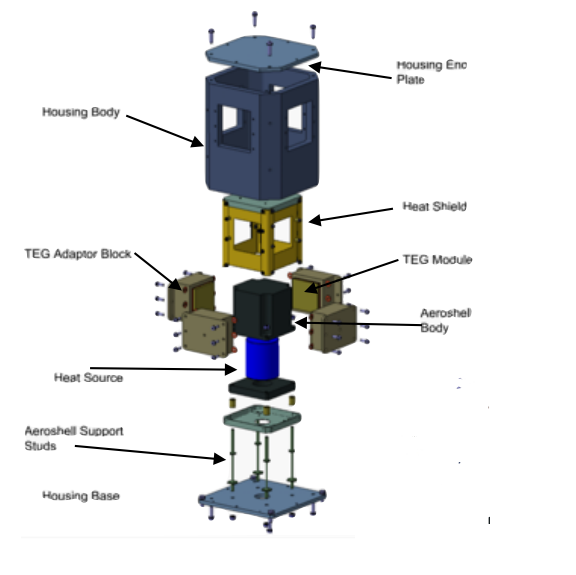
In 2012, an electrically heated breadboard experiment was conducted. The test used a cuboid form factor, rather than the more common cylindrical form, and was conducted in a liquid nitrogen cooled vacuum chamber. It was designed around a theoretical fuel element of Am2O3 which would provide 83 Wt of power to the thermocouple. This thermocouple, in the proposal phase, was either a commercially available BiTe or bespoke PbTe thermocouple, contained in a cover gas of argon. The maximum output of electrical power was 5 We, which is less than the minimum size of the “normal” 10 We RTG design. Depending on the fuel element used, it’s possible that the experiment was carried out at 83 Wt/ 5We to allow for maximum comparison between the electrically heated version and the radioisotope powered version, but the lack of RI-powered experiment data prevents us from knowing if this was the case, or whether the experiment was performed at 10 We (166 Wt?) due to manufacturing and fuel element design constraints.
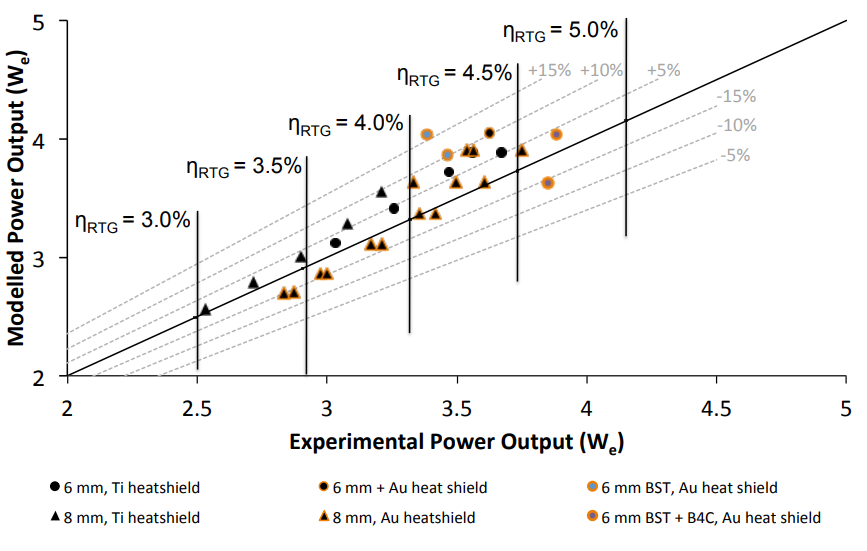
System efficiency, Ambrosi 2015 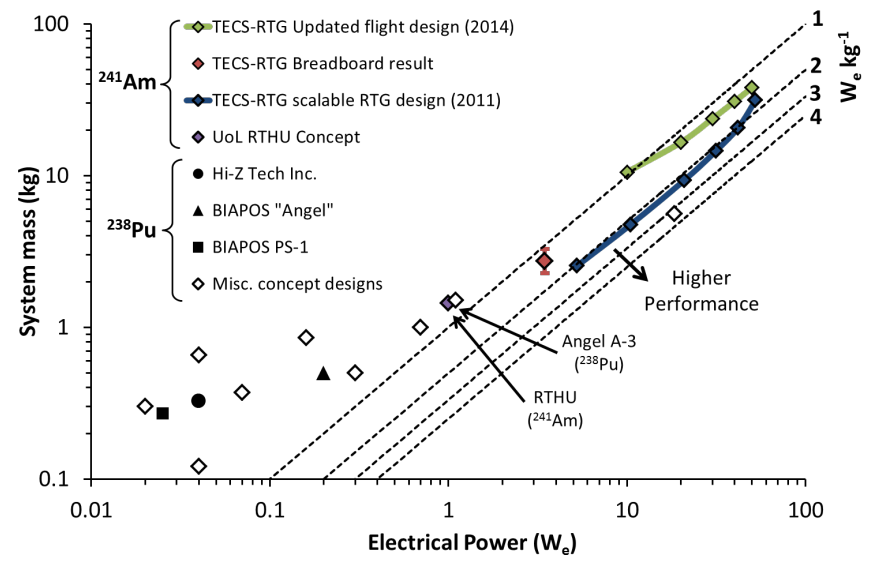
Specific Power, Ambrosi 2015
The electrically heated design in the breadboard experiment demonstrated that in the 5-50 We power output range, a specific power of 2 We/kg is feasible. There are possibilities that this could be improved somewhat, but it provides a good baseline for the power range and the specific power that these units will provide. This paper (linked below, Development and Testing of Americium-241 Radioisotope Thermoelectric Generator: Concept Designs and Breadboard System, Ambrosi et al) notes that only at small power outputs can 241Am compete with 238Pu systems, but extended mission lifetime considerations were not addressed.
ESA and the University of Leicester continue to look at expanding the use of RTGs in the future. The focus for the thermocouples in the first flight design was in bismuth telluride TEGs as of 2015. They are also looking into Stirling convertors as well, continuing in the current drive to move away from the
It takes time to do the things that they’re attempting, there are few specialists in these areas (although the fundamental tasks aren’t difficult if you know what you’re doing, it takes a while to know the ins and outs of any sort of large-scale chemistry), and it requires a lot of research to verify that each step of the way is both safe and reliable without compromising efficiency.
I have reached out to Dr. Ambrosi at University of Leicester for additional information about this test. If I hear anything from him, I will add the information about this particular test to the page on this NTR system, which should release soon.
Conclusions: 241Am, Is It the RTG Fuel of the Future?
As with most things in astronuclear engineering, the choice of an RHU fuel is a very complex question, and one which has no simple answer. 238Pu remains the preferred long-duration RTG fuel for space missions in terms of specific power, but its expense, and the requirements as far as infrastructure for fabrication and manufacture provide a high cost barrier for new entrants into the use of RHUs. For Europe, this barrier to entry is considered unacceptable, and that has kept them out of the RTG-flying world community for the entirety of their history.
241Am, on the other hand, is available to nations that conduct reprocessing of spent nuclear fuel, such as signatories to the Euratom treaty (as well as the UK, who are withdrawing voluntarily from the treaty as part of Brexit… don’t ask me why, it’s not required), where reprocessing of spent nuclear fuel is normal practice. Similar challenges to bureaucratic roadblocks to significant production of 238Pu by the US can be seen in European production of 241Am, but the existence of significant reprocessing capabilities make it theoretically far more available. 241Am is also available commercially in the US, meaning that at least in one country the regulatory barriers to possession, and therefore cost, are significantly lower than 238Pu.
This choice, however, comes at a cost of halving the specific power available to RTG systems due to the lower specific power of the fuel, at least as the design is historically described. Optimization calculations by ESA and its partners, primarily the University of Leicester, show that in the 5-50 We range of electric output the impact on mission mass is minimal, and for very low power applications (1-1.5 We) it is superior. The increased availability, and lower cost of acquisition of sufficiently pure 241Am, offset the advantages of 238Pu from the European perspective, as it integrates into current industrial capabilities, which outweigh the engineering advantages of 238Pu for the organizations involved. Even so, this is an exciting development for deep space exploration nerds, and one that can’t be overstated.
While this was a fascinating experiment, and I will be trying to find more information, the significance of this experiment boils down to one thing: this is the first time, outside the US or Russia, that an RTG designed for spacecraft use produced electrical power. It opens up new mission opportunities for ESA, who have been hampered in deep space exploration by their lack of suitable RHU fuel, and offers hope for more missions, more science, and more discoveries in the future.
References
Isotope information for 241Am, Periodictable.com https://periodictable.com/Isotopes/095.241/index.html
Isotope information for 238Pu, Periodictable.com https://periodictable.com/Isotopes/094.238/index2.full.dm.prod.html
Isotope information for 90Sr, Periodictable.com https://periodictable.com/Isotopes/038.90/index2.full.dm.prod.html
Isotope information for 210Po, Periodictable.com https://periodictable.com/Isotopes/084.210/index2.full.dm.prod.html
241Am ESA RTG Design
Development and Testing of Americium-241 Radioisotope Thermoelectric Generator: Concept Designs and Breadboard System; Ambrosi et al 2012 https://www.lpi.usra.edu/meetings/nets2012/pdf/3043.pdf
Americium-241 Radioisotope Thermoelectric Generator Development for Space Applications, Ambrosi et al 2013 https://inis.iaea.org/collection/NCLCollectionStore/_Public/45/066/45066049.pdf
Nuclear Power Sources for Space Applications – a key enabling technology (slideshow), Summerer et al, ESA 2012 https://www.euronuclear.org/events/enc/enc2012/presentations/L-Summerer.pdf
Space Nuclear Power Systems: Update on Activities and Programmes in the UK, Ambrosi (University of Leicester) and Tinsley (National Nuclear Laboratory), 2015 http://www.unoosa.org/pdf/pres/stsc2015/tech-15E.pdf




4 Responses
> [insert CERMET link]
In any case, a great post as always!
> [insert CERMET link]
In any case, a great post as always!
Fixed! Thanks for pointing that out!
Glad you liked it!